
JOURNAL
OF
CLINICAL
MICROBIOLOGY,
June
1983,
p.
1148-1152
0095-1137/83/061148-05$02.00/0
Copyright
C
1983,
American
Society
for
Microbiology
Vol.
17,
No.
6
Bacteriophage
and
Bacteriocin
Typing
Scheme
for
Clostridium
difficile
THOMAS
L.
SELL,
DENNIS
R.
SCHABERG,*
AND
F.
ROBERT
FEKETY
Division
of
Infectious
Diseases,
Department
of
Internal
Medicine,
University
of
Michigan
Medical
Center,
Ann
Arbor,
Michigan
48109
Received
1
November
1982/Accepted
3
March
1983
The
study
of
the
epidemiology
of
infection
with
Clostridium
difficile
would
be
aided
by
a
way
to
type
individual
bacterial
isolates.
We
therefore
sought
bacteriophages
for
use
in
typing.
With
mitomycin
C
exposure
(3
p.g/ml),
filtrates
from
10
strains
of
C.
difficile
had
plaque-forming
lytic
activity
on
other
C.
difficile
strains.
Individual
phage
were
passaged
and
made
into
high-titer
stock
prepara-
tions
for
typing.
Electron
microscopy
revealed
tailed
phage
particles
from
one
such
preparation.
In
addition
to
phage,
inhibitory
activity
without
distinct
plaque
formation
consistent
with
bacteriocins
was
observed
for
20
strains.
C.
difficile
isolates
from
16
patients
taken
1
to
14
days
apart
were
similar
in
their
phage
sensitivity
pattern,
whereas
isolates
from
separate
geographic
locations
showed
a
great
variety
of
patterns.
We
conclude
that
bacteriophage
should
be
useful
for
typing
strains
of
C.
difficile.
Clostridium
difficile
is
the
primary
etiological
agent
for
pseudomembranous
colitis
and
is
often
associated
with
so-called
nonspecific
cMlitis
af-
ter
antibiotic
usage.
Because
major
predisposing
factors
for
the
development
of
this
disease,
such
as
surgery
and
antimicrobial
or
cancer
chemo-
therapy,
are
common
among
hospitalized
pa-
tients,
many
cases
of
pseudomembranous
colitis
are
hospital
acquired.
Although
the
organism
is
a
strict
anaerobe,
it
forms
aerotolerant
spores
which
can
be
demonstrated
in
the
environment
about
culture-positive
patients
with
diarrhea.
The
spores
can
persist
in
the
hospital
environ-
ment
for
as
long
as
five
months
(7).
Clusters
of
pseudomembranous
colitis
have
been
described,
both
in
pediatric
and
adult
hospitalized
popula-
tions,
but
study
of
the
epidemiology
of
these
outbreaks
has
been
hindered
by
the
lack
of
a
suitable
system
for
typing
the
organisms
isolated
(5,
12;
R.
J.
Sherertz,
R.
L.
Marshall,
and
F.
A.
Sarubbi,
Program
Abstr.
Intersci.
Conf.
Antimi-
crob.
Agents
Chemother.
21st,
Chicago,
Ill.,
abstr.
no.
707,
1981).
Serotyping
has
not
been
useful
for
C.
difficile.
Bacteriophage
are
poten-
tially
useful
for
typing
and
have
been
described
with
many
clostridial
species
(1,
10).
Therefore,
we
sought
phage
active
upon
C.
difficile
for
use
in
a
typing
scheme.
MATERIALS
AND
METHODS
Bacterial
isolates.
C.
difficile
isolates
from
stool
specimens
of
different
patients
from
different
geo-
t
Present
address:
Veterans
Administration
Medical
Cen-
ter,
Division
of
Infectious
Diseases,
Allen
Park,
MI
48101.
graphic
areas
submitted
to
our
laboratory
were
select-
ed
for
study.
They
were
each
identified
by
growth
on
cycloserine-cefoxitin-fructose
agar,
biochemical
char-
acteristics,
and
fermentation
product
analysis
by
gas
liquid
chromatography
(6).
Other
Clostridia
species
were
obtained
from
the
clinical
microbiology
labora-
tory
of
the
University
of
Michigan
Hospital.
All
cul-
tures
were
grown
at
37°C
in
an
anaerobic
glove
box
(Coy
Laboratory
Products,
Ann
Arbor,
Mich.).
Lysate
production.
Isolates
were
grown
in
10
ml
of
brain
heart
infusion
(BHI)
broth
for
24
h.
After
incuba-
tion,
mitomycin
C
was
added
to
a
final
concentration
of
3
pg/ml.
After
an
additional
incubation
for
24
h,
broth
cultures
were
centrifuged
at
5,000
x
g
for
10
min,
and
the
supernatant
was
collected
after
passage
through
a
0.45-,um
membrane
filter
(Gelman
Sciences,
Inc.,
Ann
Arbor,
Mich.).
Filtrate
assay.
Filtrate
drops
were
assayed
for
lytic
or
inhibitory
activity
on
soft
agar
lawns
prepared
as
described
previously
by
Adams
(3).
BHI
agar
(1.5%)
was
used
as
the
base
with
0.75%
BHI
agar
as
an
overlay.
A
total
of
50
,ul
of
a
24-h
BHI
broth
culture
of
C.
difficile
adjusted
to
approximately
5
McFarland
units
was
used
to
seed
the
3-ml
molten
overlay.
Filtrate
drops
were
applied
to
the
cooled
soft
agar
overlay,
using
a
Steers
replicator.
Each
filtrate
was
tested
on
25
to
35
lawns
seeded
with
different
C.
difficile
isolates.
Phage
propagation.
When
plaques
were
observed,
they
were
picked
with
a
Pasteur
pipette
and
passaged
three
times
on
the
original
sensitive
lawn.
Stock
preparations
for
further
routine
testing
were
prepared
by
growing
plaques
to
near
confluence
with
the
addi-
tion
of
phage
to
the
overlay
in
the
molten
state
and
then,
after
incubation,
flooding
the
plate
with
5
ml
of
BHI
broth
and
allowing
the
flooded
plate
to
sit
for
2
to
4
h.
Decanted
broth
was
sterilized
by
passage
through
a
0.45-1m
membrane
filter
and
stored
at
4°C.
1148
PHAGE
TYPING
OF
C.
DIFFICILE
1149
Bacterial
typing.
A
bacterial
isolate
seeded
as
a
soft
agar
lawn
was
said
to
be
sensitive
to
a
phage
if
more
than
five
plaques
were
observed
with
a
standard
Steers
replicator
drop.
Isolates
with
between
one
and
five
plaques
per
drop
were
rarely
observed.
Growth
inhibition
was
said
to
occur
when
we
observed
uni-
form
decreased
growth
under
a
Steers
replicator
drop.
For
an
isolate
to
be
termed
resistant
to
a
specific
phage
or
filtrate,
the
absence
of
plaques
or
inhibition
in
the
presence
of
a
positive
control
was
necessary.
Stock
phage
preparations
were
used
at
a
concentration
that
would
produce
confluent
lysis
on
the
indicator
lawn;
for
phage
stock
in
which
confluent
lysis
could
not
be
obtained,
the
highest
available
concentration
was
used.
Electron
microscopy.
After
concentration
by
centrif-
ugation,
phage
preparations
were
placed
on
a
carbon-
coated
Formvar
grid,
negatively
stained
with
2%
phos-
photungstic
acid
at
neutral
pH,
and
examined
with
a
Philips
400
electron
microscope.
RESULTS
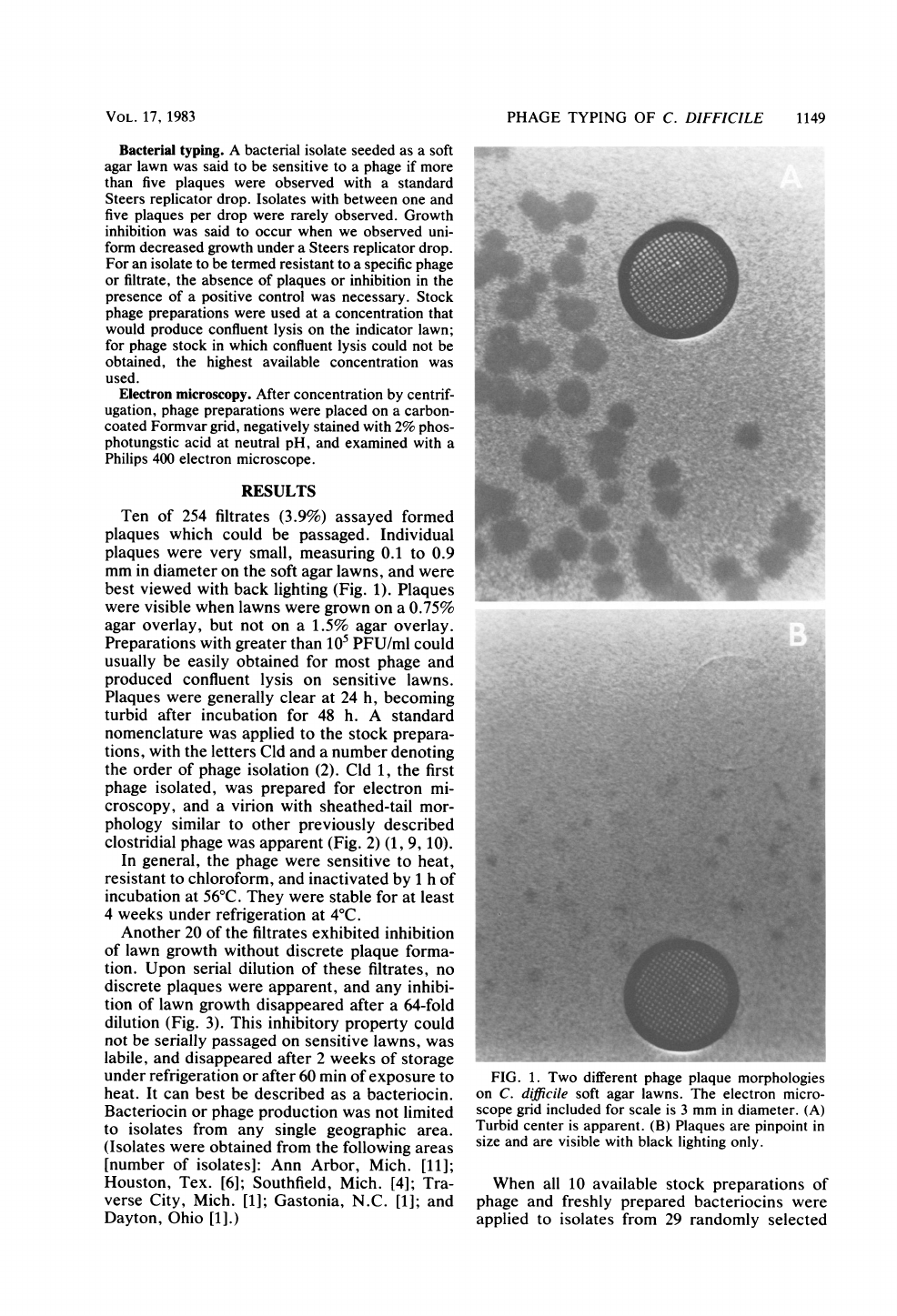
Ten
of
254
filtrates
(3.9%)
assayed
formed
plaques
which
could
be
passaged.
Individual
plaques
were
very
small,
measuring
0.1
to
0.9
mm
in
diameter
on
the
soft
agar
lawns,
and
were
best
viewed
with
back
lighting
(Fig.
1).
Plaques
were
visible
when
lawns
were
grown
on
a
0.75%
agar
overlay,
but
not
on
a
1.5%
agar
overlay.
Preparations
with
greater
than
105
PFU/ml
could
usually
be
easily
obtained
for
most
phage
and
produced
confluent
lysis
on
sensitive
lawns.
Plaques
were
generally
clear
at
24
h,
becoming
turbid
after
incubation
for
48
h.
A
standard
nomenclature
was
applied
to
the
stock
prepara-
tions,
with
the
letters
Cld
and
a
number
denoting
the
order
of
phage
isolation
(2).
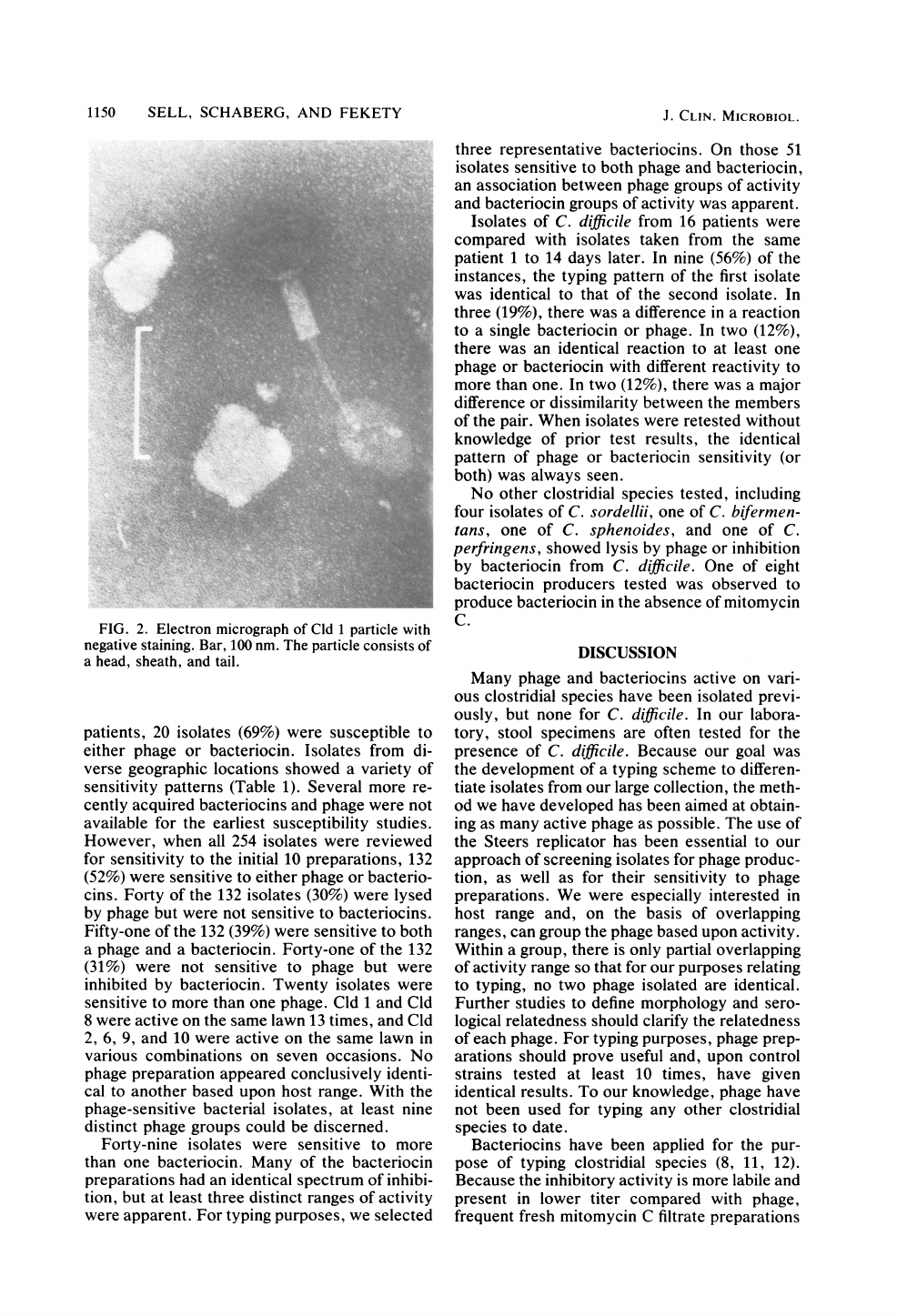
Cld
1,
the
first
phage
isolated,
was
prepared
for
electron
mi-
croscopy,
and
a
virion
with
sheathed-tail
mor-
phology
similar
to
other
previously
described
clostridial
phage
was
apparent
(Fig.
2)
(1,
9,
10).
In
general,
the
phage
were
sensitive
to
heat,
resistant
to
chloroform,
and
inactivated
by
1
h
of
incubation
at
56°C.
They
were
stable
for
at
least
4
weeks
under
refrigeration
at
4°C.
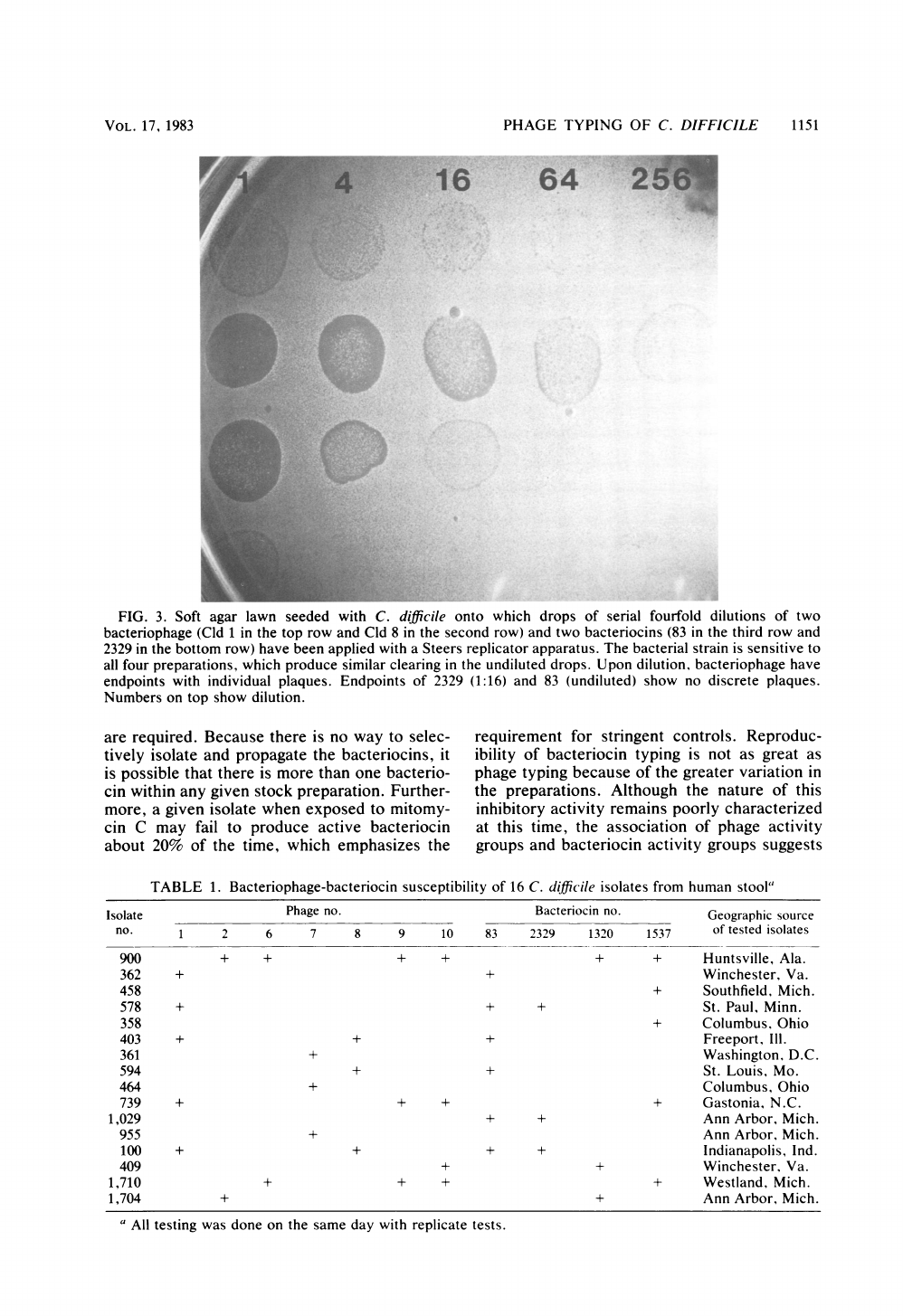
Another
20
of
the
filtrates
exhibited
inhibition
of
lawn
growth
without
discrete
plaque
forma-
tion.
Upon
serial
dilution
of
these
filtrates,
no
discrete
plaques
were
apparent,
and
any
inhibi-
tion
of
lawn
growth
disappeared
after
a
64-fold
dilution
(Fig.
3).
This
inhibitory
property
could
not
be
serially
passaged
on
sensitive
lawns,
was
labile,
and
disappeared
after
2
weeks
of
storage
under
refrigeration
or
after
60
min
of
exposure
to
heat.
It
can
best
be
described
as
a
bacteriocin.
Bacteriocin
or
phage
production
was
not
limited
to
isolates
from
any
single
geographic
area.
(Isolates
were
obtained
from
the
following
areas
[number
of
isolates]:
Ann
Arbor,
Mich.
[11];
Houston,
Tex.
[6];
Southfield,
Mich.
[4];
Tra-
verse
City,
Mich.
[1];
Gastonia,
N.C.
[1];
and
Dayton,
Ohio
[1].)
y.W-.4
.
FIG.
1.
Two
different
phage
plaque
morphologies
on
C.
difficile
soft
agar
lawns.
The
electron
micro-
scope
grid
included
for
scale
is
3
mm
in
diameter.
(A)
Turbid
center
is
apparent.
(B)
Plaques
are
pinpoint
in
size
and
are
visible
with
black
lighting
only.
When
all
10
available
stock
preparations
of
phage
and
freshly
prepared
bacteriocins
were
applied
to
isolates
from
29
randomly
selected
VOL.
17,
1983
1150
SELL,
SCHABERG,
AND
FEKETY
FIG.
2.
Electron
micrograph
of
Cld
1
particle
with
negative
staining.
Bar,
100
nm.
The
particle
consists
of
a
head,
sheath,
and
tail.
patients,
20
isolates
(69%)
were
susceptible
to
either
phage
or
bacteriocin.
Isolates
from
di-
verse
geographic
locations
showed
a
variety
of
sensitivity
patterns
(Table
1).
Several
more
re-
cently
acquired
bacteriocins
and
phage
were
not
available
for
the
earliest
susceptibility
studies.
However,
when
all
254
isolates
were
reviewed
for
sensitivity
to
the
initial
10
preparations,
132
(52%)
were
sensitive
to
either
phage
or
bacterio-
cins.
Forty
of
the
132
isolates
(30%)
were
lysed
by
phage
but
were
not
sensitive
to
bacteriocins.
Fifty-one
of
the
132
(39%)
were
sensitive
to
both
a
phage
and
a
bacteriocin.
Forty-one
of
the
132
(31%)
were
not
sensitive
to
phage
but
were
inhibited
by
bacteriocin.
Twenty
isolates
were
sensitive
to
more
than
one
phage.
Cld
1
and
Cld
8
were
active
on
the
same
lawn
13
times,
and
Cld
2,
6,
9,
and
10
were
active
on
the
same
lawn
in
various
combinations
on
seven
occasions.
No
phage
preparation
appeared
conclusively
identi-
cal
to
another
based
upon
host
range.
With
the
phage-sensitive
bacterial
isolates,
at
least
nine
distinct
phage
groups
could
be
discerned.
Forty-nine
isolates
were
sensitive
to
more
than
one
bacteriocin.
Many
of
the
bacteriocin
preparations
had
an
identical
spectrum
of
inhibi-
tion,
but
at
least
three
distinct
ranges
of
activity
were
apparent.
For
typing
purposes,
we
selected
three
representative
bacteriocins.
On
those
51
isolates
sensitive
to
both
phage
and
bacteriocin,
an
association
between
phage
groups
of
activity
and
bacteriocin
groups
of
activity
was
apparent.
Isolates
of
C.
difficile
from
16
patients
were
compared
with
isolates
taken
from
the
same
patient
1
to
14
days
later.
In
nine
(56%)
of
the
instances,
the
typing
pattern
of
the
first
isolate
was
identical
to
that
of
the
second
isolate.
In
three
(19%),
there
was
a
difference
in
a
reaction
to
a
single
bacteriocin
or
phage.
In
two
(12%),
there
was
an
identical
reaction
to
at
least
one
phage
or
bacteriocin
with
different
reactivity
to
more
than
one.
In
two
(12%),
there
was
a
major
difference
or
dissimilarity
between
the
members
of
the
pair.
When
isolates
were
retested
without
knowledge
of
prior
test
results,
the
identical
pattern
of
phage
or
bacteriocin
sensitivity
(or
both)
was
always
seen.
No
other
clostridial
species
tested,
including
four
isolates
of
C.
sordellii,
one
of
C.
bifermen-
tans,
one
of
C.
sphenoides,
and
one
of
C.
perfringens,
showed
lysis
by
phage
or
inhibition
by
bacteriocin
from
C.
difficile.
One
of
eight
bacteriocin
producers
tested
was
observed
to
produce
bacteriocin
in
the
absence
of
mitomycin
C.
DISCUSSION
Many
phage
and
bacteriocins
active
on
vari-
ous
clostridial
species
have
been
isolated
previ-
ously,
but
none
for
C.
difficile.
In
our
labora-
tory,
stool
specimens
are
often
tested
for
the
presence
of
C.
difficile.
Because
our
goal
was
the
development
of
a
typing
scheme
to
differen-
tiate
isolates
from
our
large
collection,
the
meth-
od
we
have
developed
has
been
aimed
at
obtain-
ing
as
many
active
phage
as
possible.
The
use
of
the
Steers
replicator
has
been
essential
to
our
approach
of
screening
isolates
for
phage
produc-
tion,
as
well
as
for
their
sensitivity
to
phage
preparations.
We
were
especially
interested
in
host
range
and,
on
the
basis
of
overlapping
ranges,
can
group
the
phage
based
upon
activity.
Within
a
group,
there
is
only
partial
overlapping
of
activity
range
so
that
for
our
purposes
relating
to
typing,
no
two
phage
isolated
are
identical.
Further
studies
to
define
morphology
and
sero-
logical
relatedness
should
clarify
the
relatedness
of
each
phage.
For
typing
purposes,
phage
prep-
arations
should
prove
useful
and,
upon
control
strains
tested
at
least
10
times,
have
given
identical
results.
To
our
knowledge,
phage
have
not
been
used
for
typing
any
other
clostridial
species
to
date.
Bacteriocins
have
been
applied
for
the
pur-
pose
of
typing
clostridial
species
(8,
11,
12).
Because
the
inhibitory
activity
is
more
labile
and
present
in
lower
titer
compared
with
phage,
frequent
fresh
mitomycin
C
filtrate
preparations
J.
CLIN.
MICROBIOL.
PHAGE
TYPING
OF
C.
DIFFICILE
1151
16
64
25
..
.
FIG.
3.
Soft
agar
lawn
seeded
with
C.
difficile
onto
which
drops
of
serial
fourfold
dilutions
of
two
bacteriophage
(Cld
1
in
the
top
row
and
Cld
8
in
the
second
row)
and
two
bacteriocins
(83
in
the
third
row
and
2329
in
the
bottom
row)
have been
applied
with
a
Steers
replicator
apparatus.
The
bacterial
strain
is
sensitive
to
all
four
preparations,
which
produce
similar
clearing
in
the
undiluted
drops.
Upon
dilution,
bacteriophage
have
endpoints
with
individual
plaques.
Endpoints
of
2329
(1:16)
and
83
(undiluted)
show
no
discrete
plaques.
Numbers
on
top
show
dilution.
are
required.
Because
there
is
no
way
to
selec-
tively
isolate
and
propagate
the
bacteriocins,
it
is
possible
that
there
is
more
than
one
bacterio-
cin
within
any
given
stock
preparation.
Further-
more,
a
given
isolate
when
exposed
to
mitomy-
cin
C
may
fail
to
produce
active
bacteriocin
about
20%
of
the
time,
which
emphasizes
the
requirement
for
stringent
controls.
Reproduc-
ibility
of
bacteriocin
typing
is
not
as
great
as
phage
typing
because
of
the
greater
variation
in
the
preparations.
Although
the
nature
of
this
inhibitory
activity
remains
poorly
characterized
at
this
time,
the
association
of
phage
activity
groups
and
bacteriocin
activity
groups
suggests
TABLE
1.
Bacteriophage-bacteriocin
susceptibility
of
16
C.
difficile
isolates
from
human
stool"
Isolate
Phage
no.
Bacteriocin
no.
Geographic
source
no.
1
2
6
7
8
9
10
83
2329
1320
1537
of
tested
isolates
900
+
+
+
+
+
+
Huntsville,
Ala.
362
+
+
Winchester,
Va.
458
+
Southfield,
Mich.
578
+
+
+
St.
Paul,
Minn.
358
+
Columbus,
Ohio
403
+
+
+
Freeport,
Ill.
361
+
Washington,
D.C.
594
+
+
St.
Louis,
Mo.
464
+
Columbus,
Ohio
739
+
+
+
+
Gastonia,
N.C.
1,029
+ +
Ann
Arbor,
Mich.
955
+
Ann
Arbor,
Mich.
100
+
+
+
+
Indianapolis,
Ind.
409
+
+
Winchester,
Va.
1,710
+
+
+
+
Westland,
Mich.
1,704
+
+
Ann
Arbor,
Mich.
a
All
testing
was
done
on
the
same
day
with
replicate
tests.
VOL.
17,
1983

1152
SELL,
SCHABERG,
AND
FEKETY
that
they
may
share
receptor
sites.
The
addition
of
bacteriocin
preparations
to
the
typing
scheme
that
uses
bacteriophage
increases
the
percentage
of
typable
strains
by
50%
and
is
probably
useful,
but
better
standardization
and
stabilization
of
bacteriocin
preparations
is
desirable.
Both
production
and
sensitivity
to
phage
or
bacteriocin
have been
observed
with
isolates
from
widespread
geographic
locations.
Although
it
is
likely
that
the
frequency
of
susceptibility
of
clinical
isolates
to
a
battery
of
typing
agents
could
be
increased
by
addition
of
phage
from
other
continents,
the
phages
that
are
already
available
should
be
useful
in
the
study
of
the
epidemiology
of
C.
difficile-related
disease.
We
are
continuing
to
screen
the
C.
difficile
isolates
we
have
obtained
from
patients
during
the
past
5
years.
We
have
more
than
800
isolates
that
have
not
yet
been
tested
and
have
recently
obtained
additional
apparently
new
phages.
We
are
con-
tinuing
this
work
to
improve
our
typing
system.
Typing
of
isolates
from
clusters
of
cases
of
C.
difficile
disease
should
resolve
whether
these
outbreaks
actually
represent
cross-infection
or
coincidence.
Such
information
would
directly
relate
to
the
need
for
isolation
of
culture-positive
symptomatic
patients
and
environmental
decon-
tamination.
In
addition,
since
many
patients
with
colitis
who
are
treated
will
have
a
relapse
(2),
typing
of
isolates
from
such
patients
could
differentiate
between
relapse
and
reinfection
and
assist
in
management
of
this
difficult
group
of
colitis
patients.
ACKNOWLEDGMENTS
This
work
was
supported
by
grants
from
the
Upjohn
Com-
pany
and
the
Frederick
Novy
Infectious
Diseases
Research
Fund
of
the
University of
Michigan.
We
appreciate
the
technical
assistance
of
Phyllis
Partain
and
the
electron
microscopy
of
Eugene
Minner.
LITERATURE
CITED
1.
Ackermann,
H.
1974.
Classification
of
bacteriophages
of
Bacillus
and
Clostridium.
Pathol.
Biol.
22:909-917.
2.
Ackermann,
H.,
A.
Auduviev,
L.
Berthiaume,
L.
A.
Jones,
J.
A.
Mayo,
and
A.
K.
Vidaver.
1978.
Guidelines
fe
bacteriophage
characterization.
Adv.
Virus
Res.
23:1-24.
3.
Adams,
M.
A.
1959.
Bacteriophage,
p.
443.
Interscience
Publishers,
New
York.
4.
George,
W.
L.,
N. A.
Volpicell,
D.
B.
Stiner,
D.
D.
Rich-
man,
E.
J.
Liechty,
H.
Y.
I.
Mok,
R.
D.
Rolfe,
S.
M.
Finegold.
1979.
Relapse
of
pseudomembranous
colitis
after
vancomycin
therapy.
N.
Engl.
J.
Med.
401:414-415.
5.
Greenfield,
C.,
A.
Burroughs,
M.
Szawathawski,
N.
Bass,
P.
Noone,
and
R.
Pounder.
1981.
Is
pseudo-membranous
colitis
infectious?
Lancet
i:371-372.
6.
Holdeman,
L.
V.,
E.
F.
Cato,
W.
E.
C.
Moore
(ed.).
1977.
Anaerobe
laboratory
manual,
4th
ed.,
p.
79-106.
7.
Kim,
K.
H.,
R.
Fekety,
D.
Batts,
D.
Brown,
M.
Cudmore,
J.
Silva,
and
D.
Waters.
1981.
Isolation
of
Clostridium
difficile
from
the
environment
and
contacts
of
patients
with
antibiotic-associated
colitis.
J.
Infect.
Dis.
143:42-
50.
8.
Mahoney,
D.
E.,
and
A.
Li.
1978.
Comparative
study
of
ten
bacteriocins
of
Clostridium
perfringens.
Antimicrob.
Agents
Chemother.
14:886-892.
9.
Nieves,
B.
M.,
F.
Gil,
and
F.
J.
Castillo.
1981.
Growth
inhibition
activity
and
bacteriophage
and
bacteriocinlike
particles
associated
with
different
species
of
Clostridium.
Can.
J.
Microbiol.
27:216-225.
10.
Ogata,
S.,
and
M.
Hongo.
1979.
Bacteriophages
of
the
genus
Clostridium.
Adv.
Appl.
Microbiol.
25:241-273.
11.
Satija,
K.
C.,
and
K.
G.
Navayan.
1980.
Passive
bacterio-
cin
typing
of
strains
of
Clostridium
perfringens
type
A
causing
food
poisoning
for
epidemiologic
studies.
J.
In-
fect.
Dis.
142:899-902.
12.
Tagg,
J.
R.,
A.
S.
Dajani,
and
L.
W.
Wannamaker.
1976.
Bacteriocins
of
gram-positive
bacteria.
Bacteriol.
Rev.
40:722-756.
13.
Walters,
B.,
R.
Stafford,
R.
Roberts,
and
E.
Seneviratne.
1982.
Contamination
and
crossinfection
with
C.
difficile
in
an
intensive
care
unit.
Aust.
N.Z.
J.
Med.
12:255-258.
J.
CLIN.
MICROBIOL.
